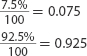The Modern Atomic Model
In the modern atomic model, electrons form an electron cloud. An electron cloud is an area around an atomic nucleus where an electron is most likely to be located. Imagine taking a time-lapse photograph of bees around a hive. You might see a blurry cloud. The cloud might be denser near the hive than farther away because the bees spend more time near the hive.
In a similar way, electrons constantly move around the nucleus. It is impossible to know both the speed and exact location of an electron at a given moment in time. Instead, scientists only can predict the likelihood that an electron is in a particular location. The electron cloud shown in Figure 10 is mostly empty space but represents the likelihood of finding an electron in a given area. The darker areas represent areas where electrons are more likely to be.
ATOM FACTS
Atoms are made up of electrons, protons and neutrons.
Atoms are made up of electrons, protons and neutrons.
Air fills most of an atom.
The nucleus of the atom is made of protons and neutrons at the center of an electron cloud.
The Parts of the Atom
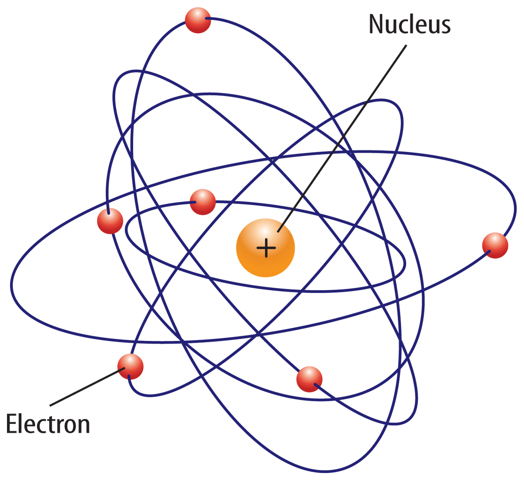
If you could see inside any atom, you probably would see the same thing—empty space surrounding a very tiny nucleus.
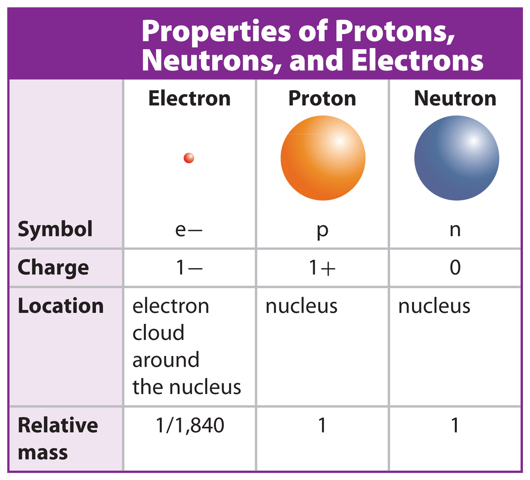
A look inside the nucleus would reveal positively charged protons and neutral neutrons. Negatively charged electrons would be whizzing by in the empty space around the nucleus.
Table 1 compares the properties of protons, neutrons, and electrons. Protons and neutrons have about the same mass. The mass of electrons is much smaller than the mass of protons or neutrons. That means most of the mass of an atom is found in the nucleus. In this lesson, you will learn that, while all atoms contain protons, neutrons, and electrons, the numbers of these particles are different for different types of atoms.
What happens to a neutral atom if it gains or loses electrons? Recall that a neutral atom has no overall charge. This is because it contains equal numbers of positively charged protons and negatively charged electrons. When electrons are added to or removed from an atom, that atom becomes an ion. An ion is an atom that is no longer neutral because it has gained or lost electrons. An ion can be positively or negatively charged depending on whether it has lost or gained electrons.
Positive Ions
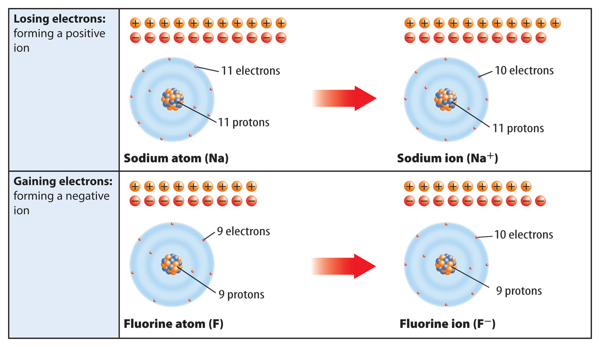
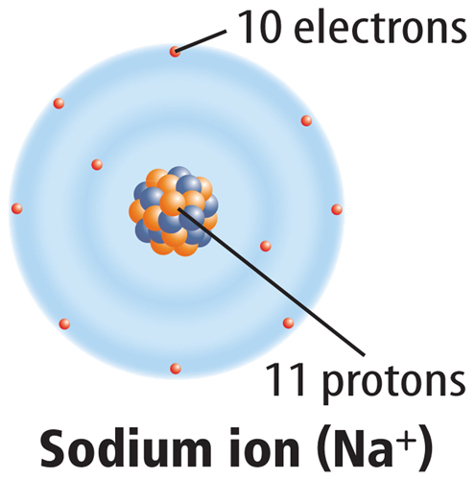
When a neutral atom loses one or more electrons, it has more protons than electrons. As a result, it has a positive charge. An atom with a positive charge is called a positive ion. A positive ion is represented by the element’s symbol followed by a superscript plus sign (+). For example, Figure 6 shows how sodium (Na) becomes a positive sodium ion (Na+).
Negative Ions
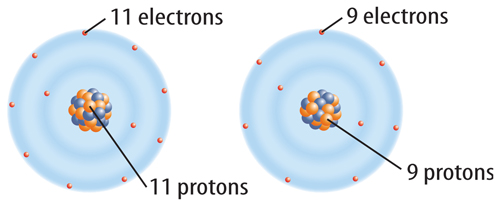
When a neutral atom gains one or more electrons, it now has more electrons than protons. As a result, the atom has a negative charge. An atom with a negative charge is called a negative ion. A negative ion is represented by the element’s symbol followed by a superscript negative sign (−). Figure 6 shows how fluorine (F) becomes a fluoride ion (F−).
1. Key Concept Check How does a neutral atom change when its number of protons, electrons, or neutrons changes?
Lesson Review
Visual Summary
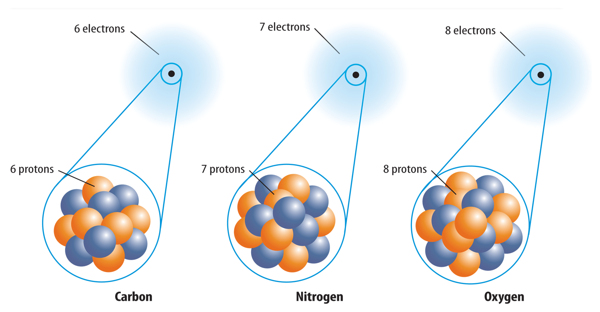
1. All atoms of the same element have the same number of protons.
2. Atoms of one element cannot be changed into atoms of another element.
3. Ions form when atoms lose or gain electrons.
Use Vocabulary
1. The number of protons in an atom of an element is its __________.
2. Nuclear decay occurs when an unstable atomic nucleus changes into another nucleus by emitting
__________.
__________.
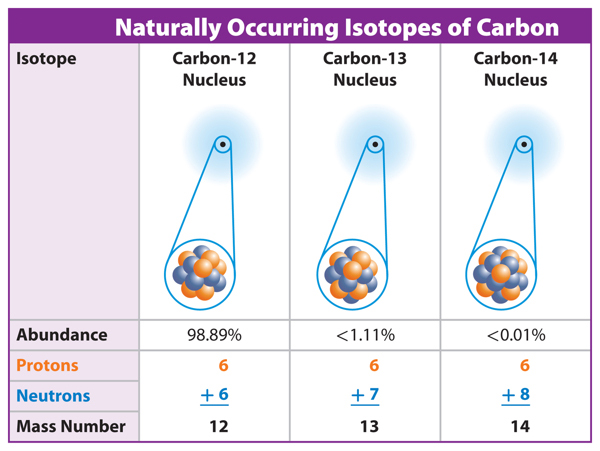
3. Describe how two isotopes of nitrogen differ from two nitrogen ions.
Understand Key Concepts
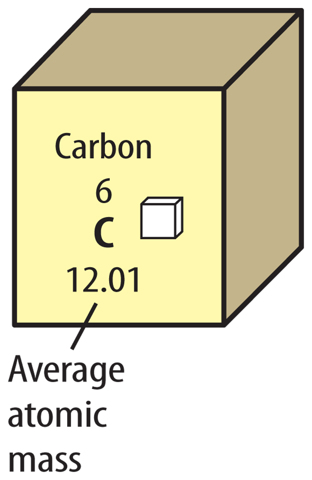
4. An element’s average atomic mass is calculated using the masses of its
A. electrons.
B. isotopes.
C. neutrons.
D. protons.
5. Compare and contrast oxygen-16 and oxygen-17.
6. Show what happens to the electrons of a neutral calcium atom (Ca) when it is changed into a
calcium ion (Ca2+).
calcium ion (Ca2+).
7. If an ion contains 10 electrons, 12 protons, and 13 neutrons, what is the ion’s charge?
A. 2−
B. 1−
C. 2+
D. 3+
8. How many neutrons does iron-59 have?
A. 30
B. 33
C. 56
D. 59
9. Which determines the identity of an element?
A. its mass number
B. the charge of the atom
C. the number of its neutrons
D. the number of its protons
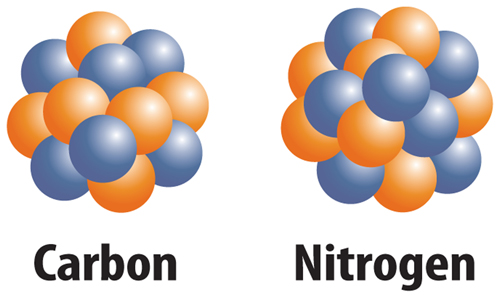
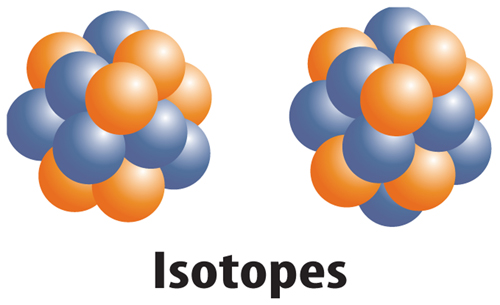
10. The figure below shows which of the following?

A. two different elements
B. two different ions
C. two different isotopes
D. two different protons
Interpret Graphics
11. Contrast Copy and fill in this graphic organizer to contrast how different elements, isotopes, and
ions are produced.
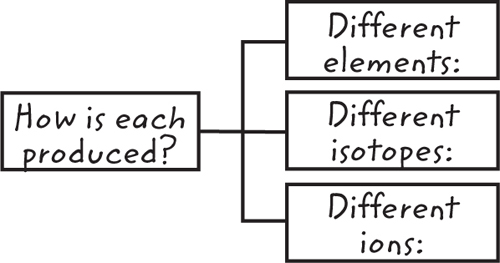
ions are produced.

Critical Thinking
12. Consider Find two neighboring elements on the periodic table whose positions would be reversed
if they were arranged by atomic mass instead of atomic number.
if they were arranged by atomic mass instead of atomic number.
13. Infer Can an isotope also be an ion?
14. Summarize how radioactive decay can produce new elements.
15. Hypothesize What might happen if a negatively charged ion comes into contact with a positively
charged ion?
charged ion?
16. Infer Why isn’t mass number listed with each element on the periodic table?
17. Explain How is the average atomic mass calculated?
18. Infer Oxygen has three stable isotopes.
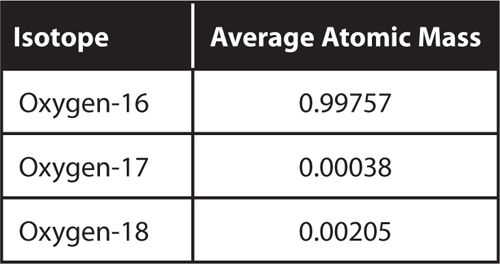
What can you determine about the average atomic mass of oxygen without calculating it?

What can you determine about the average atomic mass of oxygen without calculating it?
19. Describe the current model of the atom. Explain the size of atoms. Also explain the charge, the
location, and the size and mass of protons, neutrons, and electrons.
location, and the size and mass of protons, neutrons, and electrons.
Math Skills
Use Percentages
20. A sample of copper (Cu) contains 69.17% Cu-63. The remaining copper atoms are Cu-65. What I
is the average atomic mass of copper?
is the average atomic mass of copper?
Use the information in the table to answer questions 21 and 22.
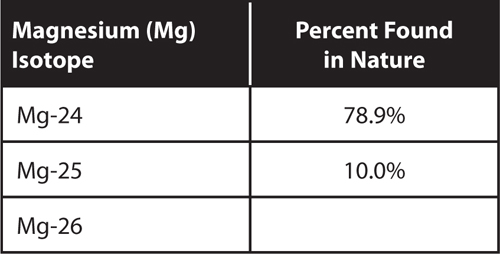

21. What is the percentage of Mg-26 found in nature?
22. What is the average atomic mass of magnesium?