Measurement and Scientific Tools
Description and Explanation
How would you describe the squirrel’s activity in Figure 1? A description is a spoken or written summary of observations. Your description might include information such as: the squirrel buried five acorns near a large tree. A qualitative description uses your senses (sight, sound, smell, touch, taste) to describe an observation. A large tree is a qualitative description. However, a quantitative description uses numbers to describe the observation. Five acorns is a quantitative description. You can use measuring tools, such as a ruler, a balance, or a thermometer, to make quantitative descriptions.
How would you explain the squirrel’s activity? An explanation is an interpretation of observations. You might explain that the squirrel is storing acorns for food at a later time. When you describe something, you report what you observe. But when you explain something, you try to interpret your observations. This can lead to a hypothesis.
The International System of Units
Suppose you observed a squirrel searching for buried food and recorded that it traveled about 200 ft from its nest. Someone who measures distances in meters might not understand how far the squirrel traveled. The scientific community solved this problem in 1960. It adopted an internationally accepted system for measurement called the International System of Units (SI).
SI Base Units and Prefixes
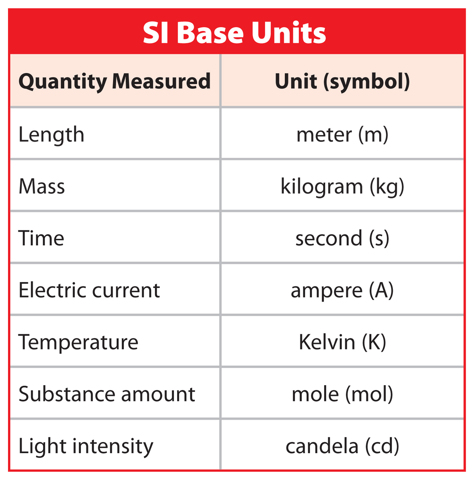
Like scientists and many others around the world, you probably use the SI system in your classroom. All SI units are derived from seven base units, as listed in Table 1. For example, the base unit for length, or the unit most commonly used to measure length, is the meter. However, you have probably made measurements in kilometers or millimeters before. Where do these units come from?
Figure 2 Scientists use the SI system when taking measurements.
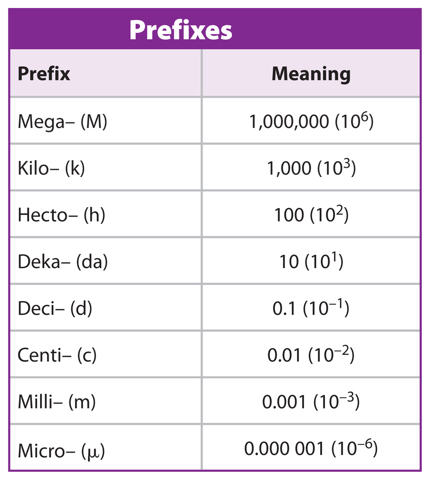
A prefix can be added to a base unit’s name to indicate either a fraction or a multiple of that base unit. The prefixes are based on powers of ten, such as 0.01 and 100, as shown in Table 2. For example, one centimeter (1 cm) is one one-hundredth of a meter and a kilometer (1 km) is 1,000 meters.
Precision and Accuracy
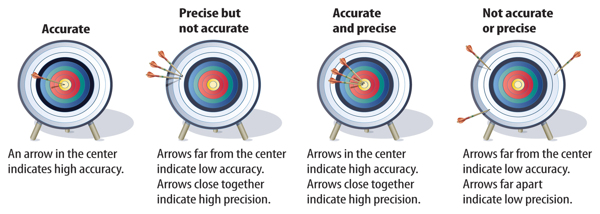
Suppose your friend Simon tells you that he will call you in one minute, but he calls you a minute and a half later. Sarah tells you that she will call you in one minute, and she calls exactly 60 seconds later. What is the difference? Sarah is accurate and Simon is not. Accuracy is a description of how close a measurement is to an accepted or true value. However, if Simon always calls about 30 seconds later than he says he will, then Simon is precise. Precision is a description of how similar or close measurements are to each other, as shown in Figure 4.
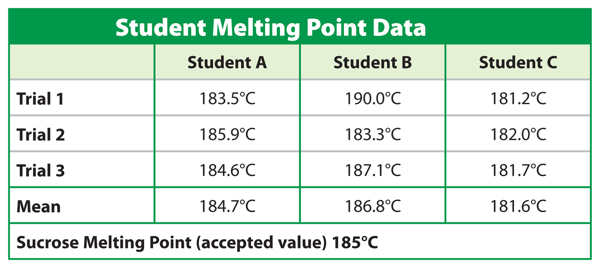
Table 3 illustrates the difference between precise and accurate measurements. Students were asked to find the melting point of sucrose, or table sugar. Each student took three temperature readings and calculated the mean, or average, of his or her data. As the recorded data in the table shows, student A had more accurate data. The melting point mean, 184.7°C, is closer to the scientifically accepted melting point, 185°C. Although not accurate, Student C’s measurements are the most precise because they are similar in value.
1. Key Concept Check How do accuracy and precision differ?
Accuracy indicates how close a measurement is to an accepted value, while precision is how close measurements are to each other.
Figure 4 The archery target illustrates accuracy and precision. An accurate shot is in the bull’s-eye.
Table 3 The data taken by student A are more accurate because each value is close to the accepted value. The data taken by student C are more precise because the data are similar.
2 NOTES
Measurement and Accuracy
Differentiated Instruction
Measurement and Accuracy
Look at a clock and make a general statement about time, such as the bell will ring soon. Discuss when the bell will ring based on what you said. Discuss whether the time you mentioned, soon, can be measured. Have students provide suggestions for measuring time with more certainty.
Guiding Questions
Why is it important for your watch to have the accurate time?

so I can get to places on time
Why is it important for a tool that measures to be calibrated to be precise and accurate?

so its measurement is precise and accurate
How can a clock be precise, but not accurate?

A clock that runs fast or slow gives consistently incorrect time, so is precise, but because the time is incorrect, it is not accurate.
Differentiated Instruction
 Precision and Accuracy Working in small groups, use tape to construct a target on the floor. Be creative. Have students toss 5 wads of paper onto the target from a set distance away. Chart each student’s precision, accuracy, and both accuracy and precision. Precision and Accuracy Working in small groups, use tape to construct a target on the floor. Be creative. Have students toss 5 wads of paper onto the target from a set distance away. Chart each student’s precision, accuracy, and both accuracy and precision.
Measurement and Accuracy
The tools used to take measurements can limit the accuracy of the measurements. Suppose you are measuring the temperature at which sugar melts, and the thermometer’s measurements are divided into whole numbers. If your sugar sample melts between 183°C and 184°C, you can estimate the temperature between these two numbers. But, if the thermometer’s measurements are divided into tenths, and your sample melts between 183.2°C and 183.3°C, your estimate between these numbers would be more accurate.
1 NOTE
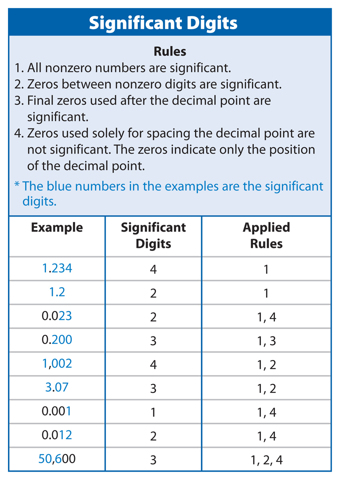
Significant Digits
Remind students that the word significant means important or essential. Direct attention to Figure 5 and read the caption. Have students estimate the distance from one wall to another in the room. Discuss the difference between an estimate and precision. Ask which would be best when ordering new carpet.
Direct attention to Table 4, read through the rules for determining significant digits, and discuss the questions.
Guiding Questions
Key Concept Check Why should you use significant digits?

to communicate the precision of the tool used to make a measurement
How many significant digits does the number 4.01 have?

3; all nonzero numbers are significant and zeros between nonzero digits are significant.
Why is it important to consider all four rules when determining significant digits?

to be as accurate as possible
Significant Digits
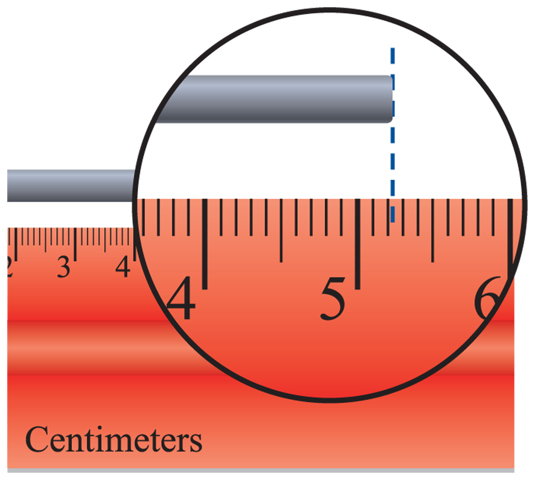
In the second example above, you know that the temperature is between 183.2°C and 183.3°C. You could estimate that the temperature is 183.25°C. When you take any measurement, some digits you know for certain and some digits you estimate. Significant digits are the number of digits in a measurement that are known with a certain degree of reliability. The significant digits in a measurement include all digits you know for certain plus one estimated digit. Therefore, your measurement of 183.25°C would contain five significant digits, as explained in Table 4. Using significant digits lets others know how certain your measurements are. Figure 5 shows an example of rounding to 3 significant figures.
1. Key Concept Check Why should you use significant digits?
to communicate the precision of the tool used to make a measurement
Figure 5 Since the ruler is divided into tenths, you know the rod is between 5.2 cm and 5.3 cm. You can estimate that the rod is 5.25 cm.
3 NOTES
Differentiated Instruction
Differentiated Instruction
Math Activity
Differentiated Instruction
 Significant Digits Give students the following examples: a school with a student population of 1500; a city with a population of 150,000; a country with a population of 15,000,000. Ask them to determine the number of significant digits in each example and to state which rules they used to make their determinations. (two; rules 1 and 4) Ask them to explain how each of these numbers can have the same number of significant digits, yet represent vastly different quantities. (The numbers reflect the degree of precision with which you can measure each population. For example, the population of a city might be known to the nearest thousand, while the population of a country might be known only to the nearest million.) Significant Digits Give students the following examples: a school with a student population of 1500; a city with a population of 150,000; a country with a population of 15,000,000. Ask them to determine the number of significant digits in each example and to state which rules they used to make their determinations. (two; rules 1 and 4) Ask them to explain how each of these numbers can have the same number of significant digits, yet represent vastly different quantities. (The numbers reflect the degree of precision with which you can measure each population. For example, the population of a city might be known to the nearest thousand, while the population of a country might be known only to the nearest million.)
Differentiated Instruction
 Vocabulary Aids Have students use index cards and markers. Vocabulary Aids Have students use index cards and markers.
1. Have students draw and label a picture to demonstrate accuracy.
2. Repeat the activity for precision and significant digits.
3. Have students punch a hole in the corner of the cards and use a large paperclip or ring to hold them together.
Math Activity
Significant Digits Use the following activity to help students gain a better understanding of significant digits.
1. Prepare the following numerals on the board or chart paper: 4.05, 770.032, .0025. Discuss which numbers are significant.
2. Prepare a series of numerals written on chart paper.
3. Underline each number that is a significant digit.
4. Cover each numeral with a sticky note containing the same numeral without the underlines.
5. Ask students to read the digits and determine which numerals are significant. Remove the sticky notes to check answers.
6. Ask students to write three numerals each containing a decimal point. Students should design an answer key to identify the significant digits for each numeral. Check for understanding and correct application. Students might exchange numerals for added practice.
Math Skills: Significant Digits
The number 5,281 has 4 significant digits. Rule 1 in Table 4 above states that all nonzero numbers are significant.
1. Practice
Use the rules in Table 4 to determine the number of significant digits in each of the following numbers: 2.02; 0.0057; 1,500; and 0.500.
2.02—3 significant digits; 0.0057—2 significant digits; 1,500—2 significant digits; 0.500—3 significant digits
1 NOTE
SCIENCE USE V. COMMON USE
digital
Ask: Do you prefer to use a digital clock or an analog clock?
Answers will vary.
Ask: How many digits do you have on one foot or one hand?
five digits on each
SCIENCE USE V. COMMON USE
digital
Science Use of, pertaining to, or using numbers (numerical digits)
Common Use of or pertaining to a finger or toe
10 NOTES
Scientific Tools Science Journal / Balances
Thermometer
Glassware
Compound Microscope
Computers — Hardware and Software
Differentiated Instruction
Differentiated Instruction
Differentiated Instruction
Teacher Demo
Reading Strategy
Scientific Tools
Science Journal / Balances
Have students read each paragraph. Use the following questions to help students understand the purpose and use of each tool.
Guiding Questions
What is the purpose of a science journal?

to record data, observations, and other important information
Why is it important to keep a science journal?

to have a neat, organized, clearly written, accurate, and thorough record of your procedures, questions, and results
What is the purpose of a balance?

to measure the mass of objects
Why is it important to carefully place objects on a balance?

so the object is not dropped onto the pan, possibly damaging the precision and accuracy of the balance
Thermometer
After discussing the following questions, brainstorm with students to find situations when they might use thermometers.
Guiding Questions
What does a thermometer measure?

the temperature of substances
Why should a thermometer never be used as a stirring rod?

It might break. It might give an inaccurate reading.
What safety precautions should you take when handling a glass thermometer?

handle it carefully and do not let it roll off the table, lower it into hot substances with care so you don’t get burned, report to the teacher if the thermometer breaks
Glassware
Show students examples of glassware, such as flasks, beakers, petri dishes, test tubes, and graduated cylinders. Discuss the following questions.
Guiding Questions
What are graduated cylinders used to measure?

the volume of liquids
What unit is used for measuring volume?

usually liters and milliliters
What is the purpose of laboratory glassware?

to hold, pour, heat, and measure liquids
Why is a beaker not considered an accurate tool of measurement?

Because of their wide base, measurements can not be as accurate as with a slender graduated cylinder.
Compound Microscope
Use the Reference Handbook to identify the parts of a compound microscope and demonstrate how to use it.
Guiding Questions
Why is a microscope used?

to see small objects that cannot be seen with an unaided eye
What precautions should be followed when moving a microscope?

carry it carefully by holding the arm of the microscope with one hand and supporting the base with the other hand
What is something that could be viewed with a microscope?

Accept all reasonable responses. Encourage students to think of interesting things to view that can only be seen by using a microscope. Possible answer: bacteria, cells, dust
Computers—Hardware and Software
Many students are familiar with computers, but some students may not be aware of the vast and varied amount of data that computers can analyze. Have students read the two paragraphs. Discuss with them the guided questions. Have them suggest examples of how computers can help a scientist study an organism.
Guiding Questions
What is the difference between computer hardware and computer software?

hardware—the main components of the computer; software—the computer programs
How do computers help scientists interpret information and data?

Possible answer: Scientists can use computers to compare and analyze data, research information, and communicate with fellow scientists.
Differentiated Instruction
 Safety First! Divide the class into student pairs. Using paper and markers, have students determine one safety rule for one scientific tool. Students should use their rules to create a Safety First! cartoon. Display the cartoons around the room. Safety First! Divide the class into student pairs. Using paper and markers, have students determine one safety rule for one scientific tool. Students should use their rules to create a Safety First! cartoon. Display the cartoons around the room.
Differentiated Instruction
 Scientific Tools Divide the class into small groups. Have each group design a game using index cards. For example, some groups could design a card game, in which the name of the tool is written on one card, its purpose on a second card, and its units on a third card. Have students shuffle the cards and deal three cards to each player. The object would be to create matching sets. Students may ask an opponent if he or she has a particular card or may draw from the deck to look for matches. The winner could be the first student to make a set, or the student who makes the most sets. Scientific Tools Divide the class into small groups. Have each group design a game using index cards. For example, some groups could design a card game, in which the name of the tool is written on one card, its purpose on a second card, and its units on a third card. Have students shuffle the cards and deal three cards to each player. The object would be to create matching sets. Students may ask an opponent if he or she has a particular card or may draw from the deck to look for matches. The winner could be the first student to make a set, or the student who makes the most sets.
Differentiated Instruction
 Vocabulary Aids Have students add to their study cards for the lesson. Vocabulary Aids Have students add to their study cards for the lesson.
1. Have students draw a picture and label each scientific tool—one scientific tool per card.
2. Have students write the use of each scientific tool on the back of each card.
3. Have students pair up to discuss the cards they have created and take turns explaining the use of each scientific tool.
Teacher Demo
Scientific Tools
1. Gather the scientific tools described in this section.
2. Demonstrate proper use of each tool.
3. Discuss the safety measures needed for use with each tool.
4. Provide opportunities for students to use the scientific tools to familiarize themselves with them.
Reading Strategy
Taking Notes Write the name of one of the scientific tools on the board or chart paper. Redirect students to the paragraph explaining the scientific tool in the lesson. Have students reread the information about the scientific tool and identify the main points. Create a bulleted list under the name of the tool as students identify each main point. Have students practice this note-taking technique using the paragraphs describing other scientific tools. You may want to divide the class into groups, have them complete the task for an assigned tool, and then share their bulleted lists.
Scientific Tools
Scientific inquiry often requires the use of tools. Scientists, including life scientists, might use the tools listed below. You might use one or more of them during a scientific inquiry, too. For more information about the proper use and care of microscopes, see the Reference Handbook. For more information on other scientific tools, see the Science Skill Handbook.
Science Journal
In a science journal, you can record descriptions, explanations, plans, and steps used in a scientific inquiry. A science journal can be a spiral-bound notebook or a loose-leaf binder. It is important to keep your science journal organized so that you can find information when you need it. Make sure you keep thorough and accurate records.
Balances
You can use a triple-beam balance or an electric balance to measure mass. Mass usually is measured in kilograms (kg) or grams (g). When using a balance, do not let objects drop heavily onto the balance. Gently remove an object after you record its mass.
Thermometer
A thermometer measures the temperature of substances. Although the Kelvin (K) is the SI unit for temperature, in the science classroom, you measure temperature in degrees Celsius (°C). Use care when you place a thermometer into a hot substance so that you do not burn yourself. Handle glass thermometers gently so that they do not break. If a thermometer does break, tell your teacher immediately. Do not touch the broken glass or the thermometer’s liquid. Never use a thermometer to stir anything.
Glassware
Laboratory glassware is used to hold, pour, heat, and measure liquids. Most labs have many types of glassware. For example, flasks, beakers, petri dishes, test tubes, and specimen jars are used as containers. To measure the volume of a liquid, you use a graduated cylinder. The unit of measure for liquid volume is the liter (L) or milliliter (mL).
Compound Microscope
Microscopes enable you to observe small objects that you cannot observe with just your eyes. Usually, two types of microscopes are in science classrooms—dissecting microscopes and compound light microscopes, such as the one shown below. The girl is looking into two eyepieces to observe a magnified image of a small object or organism. However, some microscopes have only one eyepiece.
Microscopes can be damaged easily. It is important to follow your teacher’s instructions when carrying and using a microscope. For more information about how to use a microscope, see the Reference Handbook.
Computers—Hardware and Software
Computers process information. In science, you can use computers to compile, retrieve, and analyze data for reports. You also can use them to create reports and other documents, to send information to others, and to research information.
The physical components of computers, such as monitors and keyboards, are called hardware. The programs that you run on computers are called software. These programs include word processing, spreadsheets, and presentation programs. When scientists write reports, they use word processing programs. They use spreadsheet programs for organizing and analyzing data. Presentation programs can be used to explain information to others.
1 NOTE
Magnifying Lens, Slide, Dissecting Tools, Pipette
Discuss the information in each paragraph as each one is read. Direct students to the images of the different tools as you discuss the type of tools used by life scientists. If possible, show and demonstrate use of each tool.
Guiding Questions
What is the purpose of a magnifying lens?

to enlarge the image of an object
Why must slides be carefully handled?

because they are made of glass, easily broken, and could cut you
Key Concept Check What are some tools used by life scientists?

Accept all reasonable responses.
Tools Used by Life Scientists
Magnifying Lens
Slide
Lesson AssessmentUse Vocabulary
1. Define description and explanation in your own words.
Sample answer: A description is a summary of observations and an explanation is an interpretation of those observations.
2. Use the term International System of Units (SI) in a sentence.
Sample answer: The International System of Units is a system for measurement that is used and accepted by scientists worldwide.
Understand Key Concepts
3. Which tool would a scientist use to view a tiny organism?
A. computer
B. compound microscope
C. test tube
D. triple-beam balance
4. Describe the difference between accuracy and precision.
Accuracy is a description of how close a measurement is to an accepted value. Precision is a description of how similar or close measurements are to each other.
5. Explain why scientists use significant digits.
Scientists use significant digits to communicate the precision of their measurements to others.
6. Which is a quantitative observation?
A. 15 m long
B. red color
C. rough texture
D. strong odor
7. Which is one way scientists indicate how precise and accurate their experimental measurements
are?
A. They keep accurate, honest records.
B. They make sure their experiments can be repeated.
C. They use significant figures in their measurements.
D. They record small samples of data.
Interpret Graphics
8. Draw a graphic organizer like the one below. Write the name of an SI base unit in each circle. Add additional circles to the graphic organizer as needed.
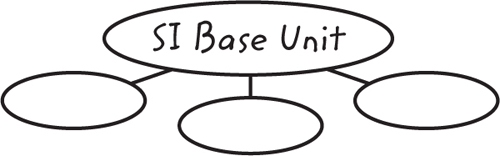
The graphic organizer should have seven circles around the center circle. The center circle should say, SI Base Units. The following terms should be in the individual circles, in any order, surrounding the center circle: length, mass, time, electric current, temperature, substance amount, light intensity.
Critical Thinking
9. Recommend ways that computers can assist life scientists in their work.
Answers should recommend ways in which computers can assist life scientists in their work. Sample answer: Life scientists use a computerized spreadsheet to keep track of their data, research information, graph data, and exchange ideas with scientific peers.
Math Skills
10. Suppose you measure the mass of a book and it is 420.0890 g. How many significant digits are
in this measurement?
7 significant digits
11. How many significant figures are in 0.00840, 15.7, and 13.040?
3; 3; 5
Copyright © 2011-2012 The McGraw-Hill Companies. All Rights Reserved.
Texas Essential Knowledge and Skills Copyright © 2011. Texas Education Agency. All Rights Reserved. |
2010 - MarshScience7 is proudly powered by Blogger
Blogger Template created by Anshul
Design By Templatelite.com
Blogger Template created by Anshul
Design By Templatelite.com












0 comments:
Post a Comment