Game Review
The Carbon Cycle Game Quiz
Video
A Diamond?
The yellow rock probably does not look like a diamond that you have seen. It lacks sparkle because it is rough and uncut. It formed deep within Earth under intense pressure and heating. Believe it or not, diamonds are pure carbon.
Elements in Living Things
What do you have in common with the fish and sea anemones in Figure 1? You might be surprised to learn that you, a fish, and a sea anemone have several things in common. Each of you is made of cells that contain carbon, hydrogen, oxygen, nitrogen, and a few other elements. In fact, the mass of all living organisms contain about 18 percent carbon compounds.
Except for water and some salts, most things you put in or on your body—food, clothing, cosmetics, and medicines—consist of compounds that contain carbon. This lesson explores various types of carbon compounds that make up living things.
1.  Reading Check Which four elements are in most living organisms?
Reading Check Which four elements are in most living organisms?
 Reading Check Which four elements are in most living organisms?
Reading Check Which four elements are in most living organisms?
Organic Compounds
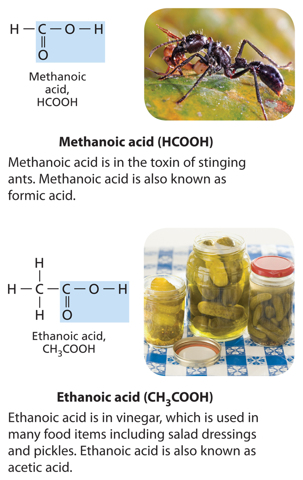
Scientists once thought that all carbon compounds came from living or once-living organisms, and they called these compounds organic. Scientists now know that carbon is also in many nonliving things. Today, scientists define an organic compound as a chemical compound that contains carbon atoms usually bonded to at least one hydrogen atom. Organic compounds can also contain other elements such as oxygen, nitrogen, phosphorus, or sulfur. However, compounds such as carbon dioxide (CO2) and carbon monoxide (CO) are not organic because they do not have a carbon-hydrogen bond.
Understanding Carbon
A carbon atom is unique because it can easily combine with other atoms and form millions of compounds. Find carbon on the periodic table in the Reference Handbook. Carbon has an atomic number of 6. Therefore, a neutral carbon atom has six protons and six electrons. Four of these electrons are valence electrons, or are in the outermost energy level. Recall that many atoms are chemically stable when they have eight valence electrons. Carbon atoms become more chemically stable through covalent bonding, as shown in Figure 2. In a covalent bond, carbon atoms have eight valence electrons, like a stable, unreactive noble gas.
The Carbon Group
Look again at the periodic table. Notice that silicon and germanium are in the same group as carbon. They each have four valence electrons. Silicon and germanium atoms also become stable by forming four covalent bonds. However, it takes more energy for them to do this. The more energy it takes, the less likely it is that bonding will occur.
1. Key Concept Check How is carbon unique compared to other elements?
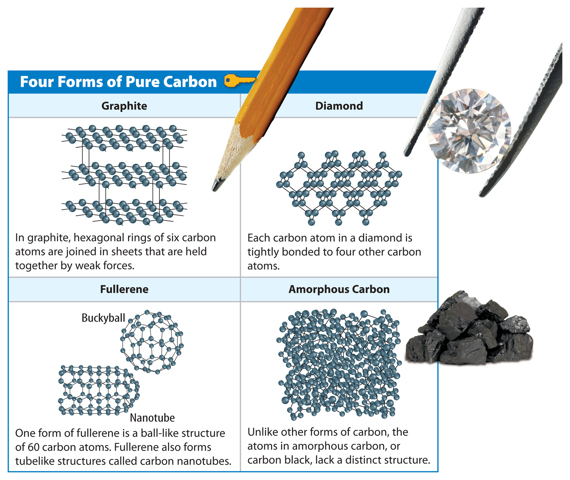
The Forms of Pure Carbon
Figure 3 Four common forms of pure carbon are graphite, diamond, fullerene, and amorphous.
 Visual Check Compare the structures of graphite and diamonds and explain why graphite is used in pencil lead but diamonds are not.
Visual Check Compare the structures of graphite and diamonds and explain why graphite is used in pencil lead but diamonds are not.
When carbon atoms bond together, they form one of several different arrangements, such as those shown in Figure 3. Forms of carbon are described below.
• One form of carbon is graphite (GRA fite). In graphite, carbon atoms form thin sheets that can slide over one another or bend. Graphite is used as a lubricant and in making golf clubs, tennis rackets, pencil lead, and other items.
• Diamonds, another form of carbon, are used in jewelry, on the tips of drill bits, and on the edges of some saw blades. The carbon atoms bond to one another in a rigid and orderly structure, making diamonds extremely strong. This makes diamonds one of the hardest materials known.
• Carbon atoms in fullerene (FOOL uh reen) form various cagelike structures. Fullerene was discovered late in the twentieth century and uses for fullerene are still being explored. However, future fullerene uses might include the development of faster, smaller electronic components.
• The atoms in amorphous (uh MOR fus) carbon lack an orderly arrangement. Amorphous carbon is found in soot, coal and charcoal.
1
SCIENCE USE V. COMMON USE
organic
Science Use a chemical compound that contains carbon and usually contains at least one carbon-hydrogen bond
Science Use a chemical compound that contains carbon and usually contains at least one carbon-hydrogen bond
Common Use pertaining to or grown with natural fertilizers and pesticides; often used to refer to foods, such as fruits, vegetables, and meats
1
REVIEW VOCABULARY
covalent bond
a chemical bond formed by sharing one or more pairs of electrons between atoms
a chemical bond formed by sharing one or more pairs of electrons between atoms
1
WORD ORIGIN
amorphous
from a– and Greek morphe, means “without form”
from a– and Greek morphe, means “without form”
21
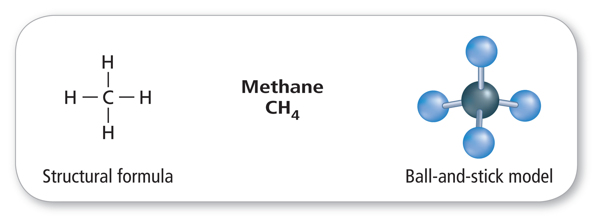
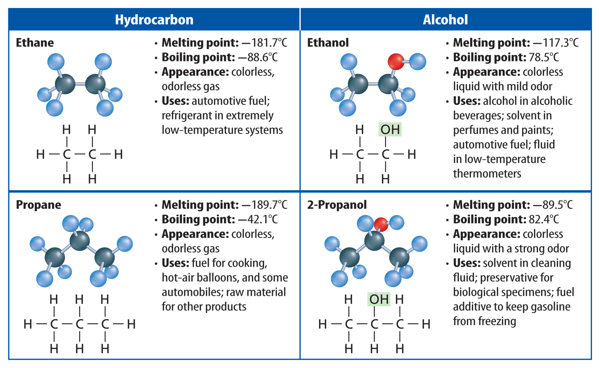
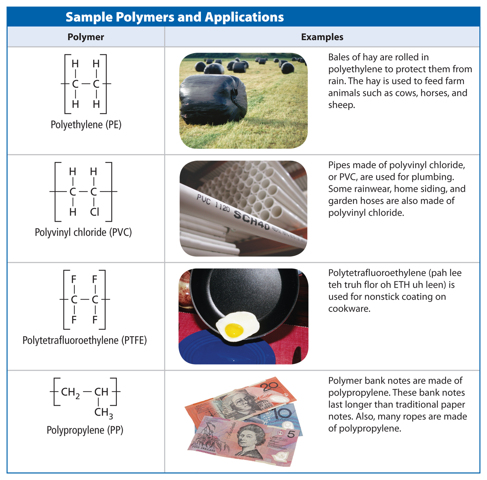
Hydrocarbons
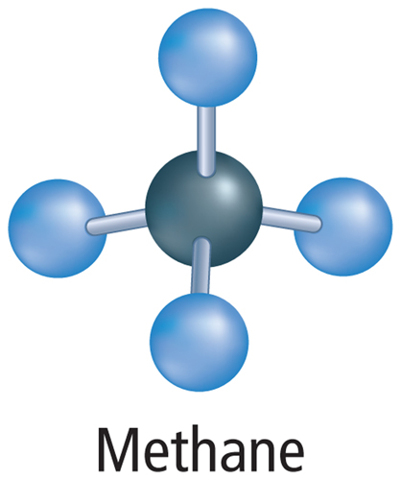
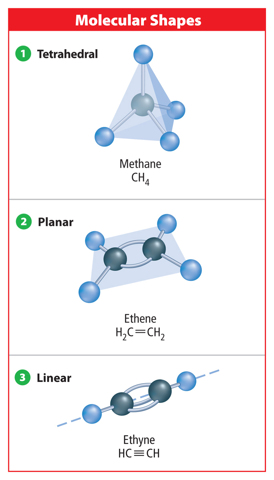
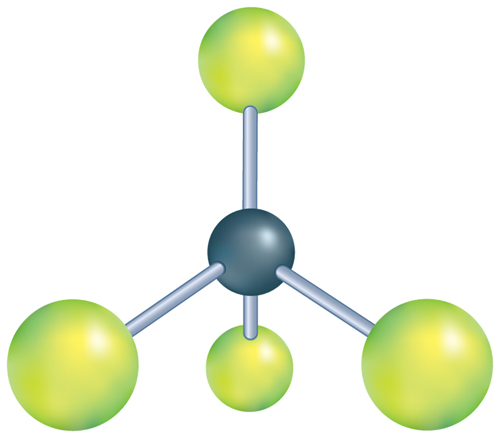
Many organic compounds contain only carbon and hydrogen atoms, however, organic compounds can also contain other elements such as oxygen, phosphorus, nitrogen, or sulfur. A compound that contains only carbon and hydrogen atoms is called a hydrocarbon. There are many different hydrocarbons. The simplest is methane (CH4), shown in Figure 4.
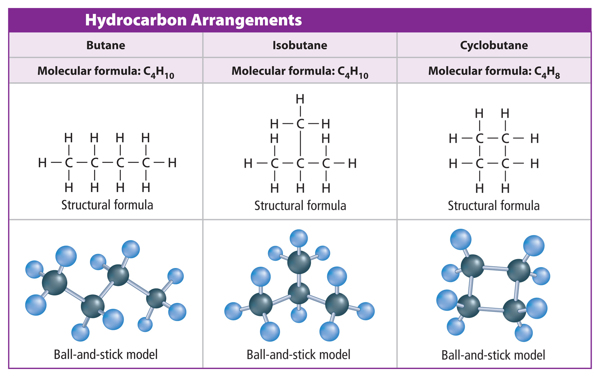
Hydrocarbon Chains
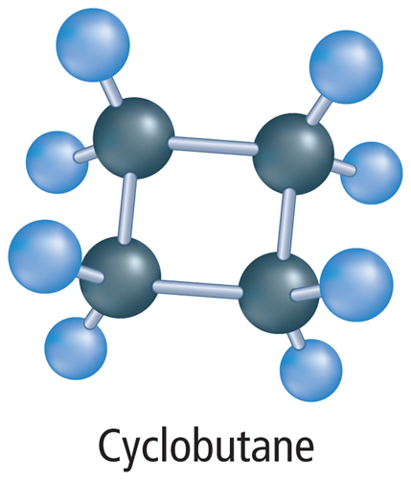
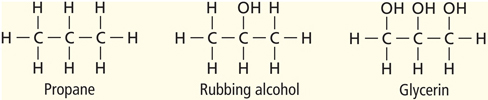
When carbon atoms form hydrocarbons, the carbon atoms can link together in different ways. They can form straight chains, branched chains, or rings. Table 1 shows examples of each type of arrangement. Look closely at the molecular formula for each compound. Notice that butane and isobutane have the same molecular formula. They have the same ratio of carbon atoms to hydrogen atoms. Compounds that have the same molecular formula but different structural arrangements are called isomers (I suh murz). Each isomer is a different molecule with its own unique name and properties.
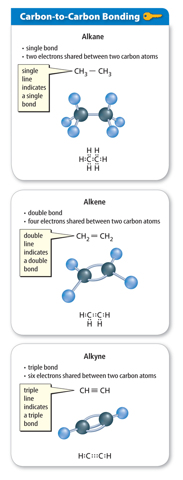
Carbon-to-Carbon Bonding
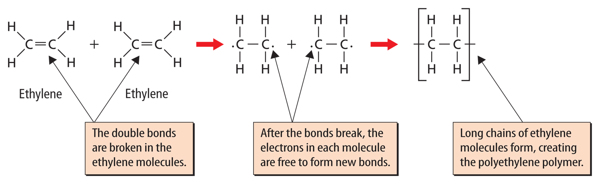
When a carbon atom bonds to another carbon atom, the two atoms can share two, four, or six electrons, as shown in Figure 5. In all cases, the carbon atoms in each molecule have eight valence electrons and are stable. When two carbon atoms share two electrons, it is called a single bond. A hydrocarbon that contains only single bonds is called an alkane. When two carbon atoms share four electrons, it is called a double bond. A hydrocarbon that contains at least one double bond is called an alkene. If two carbon atoms share six electrons, it is called a triple bond. A hydrocarbon that contains at least one triple bond is called an alkyne.
1. Key Concept Check What kind of bonds do carbon atoms form with other carbon atoms?
Saturated Hydrocarbons
Hydrocarbons are often classified by the type of bonds the carbon atoms share. A hydrocarbon that contains only single bonds is called a saturated hydrocarbon. It is called saturated because no more hydrogen atoms can be added to the molecule. Look at the top image in Figure 5. Notice that three of the valence electrons in each carbon atom bond with hydrogen atoms. The carbon atoms are saturated with hydrogen atoms.
Unsaturated Hydrocarbons
A hydrocarbon that contains one or more double or triple bonds is called an unsaturated hydrocarbon. Look at the double bond and triple bond examples in Figure 5. If the double and triple bonds are broken, additional hydrogen atoms could bond to the carbon atoms. Therefore, molecules containing double and triple bonds are not saturated with hydrogen atoms.
2.  Reading Check Explain the difference between saturated and unsaturated compounds.
Reading Check Explain the difference between saturated and unsaturated compounds.
 Reading Check Explain the difference between saturated and unsaturated compounds.
Reading Check Explain the difference between saturated and unsaturated compounds.
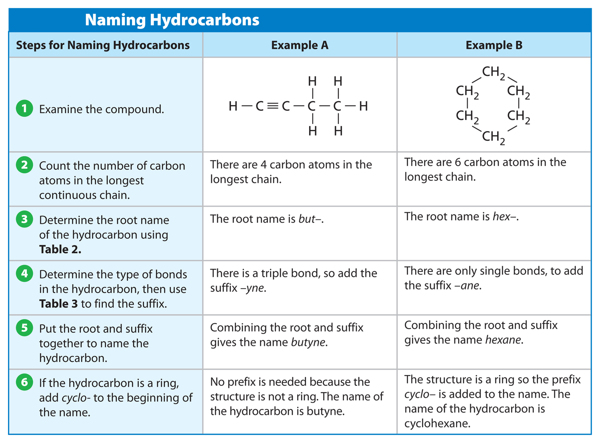
Naming Hydrocarbons
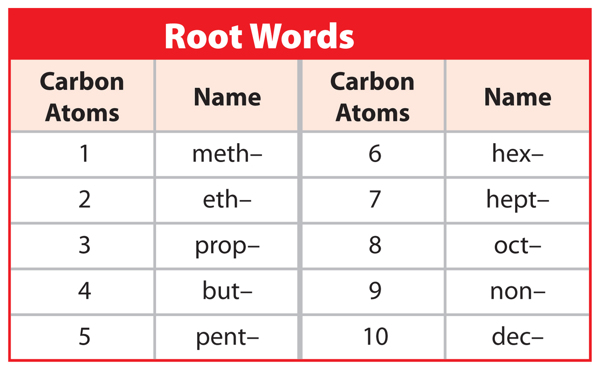
What type of shape is a stop sign? It is an octagon. An octagon is a figure with eight sides. Its name comes from the root oct–, which means “eight.” Most geometric shapes have names that refer to the number of sides they have, such as a triangle. Similarly, hydrocarbons have names that indicate how many carbon atoms are in each molecule.
Carbon Chains
When naming a hydrocarbon, the first thing you need to do is find the longest carbon chain and count the number of carbon atoms in it. Look at Figure 6. Find the carbon chain and count the carbon atoms. In this molecule, there are four carbon atoms. The number of carbon atoms gives you the root word of the name. Now, look at Table 2. This table shows the root word for any hydrocarbon that has one through ten carbon atoms. What is the root name for the molecule in Figure 6? The root name is but– (BYEWT). What would be the root name if the carbon chain had eight carbon atoms like a stop sign? The root name is oct–.
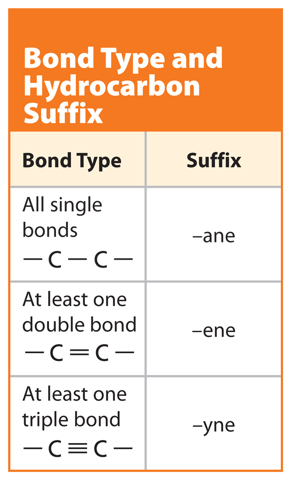
Determine the Suffix
Now that you know how to find the root word of a hydrocarbon, you must also find the suffix, or end, of the name. Recall that carbon atoms bond to other carbon atoms by single bonds, double bonds, or triple bonds. Table 3 shows which suffix to use when the type of bonds in the molecule are determined. Look at Figure 6 again. The molecule has all single bonds and should have the suffix –ane. Put the root and the suffix together and you get butane.
3.  Reading Check What is the name of a hydrocarbon with six carbon atoms that contains only single bonds?
Reading Check What is the name of a hydrocarbon with six carbon atoms that contains only single bonds?
 Reading Check What is the name of a hydrocarbon with six carbon atoms that contains only single bonds?
Reading Check What is the name of a hydrocarbon with six carbon atoms that contains only single bonds?
Determine the Prefix
Sometimes hydrocarbons have a prefix, and sometimes they do not. Recall that hydrocarbons form chains, branched chains, and rings. If a hydrocarbon contains a ring structure, the prefix cyclo– is added before the root name. Hydrocarbons sometimes have other prefixes and numbers added before their name. You might read about this naming system in more advanced chemistry courses. For this lesson, only hydrocarbons in the form of a ring will get a prefix. Table 4 summarizes the steps used to name a hydrocarbon.
Lesson Review
Visual Summary
Chemical compounds that contain carbon and usually at least one hydrogen atom are called organic compounds.
Carbon atoms form several different substances including graphite, diamonds, fullerene, and amorphous carbon.
A hydrocarbon can be in the form of a straight chain, branched chain, or a ring structure.
What do you think NOW?
You first read the statements below at the beginning of the lesson.
1. Charcoal and diamonds are made of carbon atoms.
2. Carbon atoms often bond with hydrogen atoms in chemical compounds.
Did you change your mind about whether you agree or disagree with the statements? Rewrite any false statements to make them true.
Lesson Assessment
Use Vocabulary
1. Use the Word Use the words organic compound in a sentence.
2. Define saturated hydrocarbon and unsaturated hydrocarbon.
3. Use the term isomer in a complete sentence.
Understand Key Concepts
4. List Besides carbon, what other elements can organic compounds contain?
5. Summarize how carbon bonds with other carbon atoms.
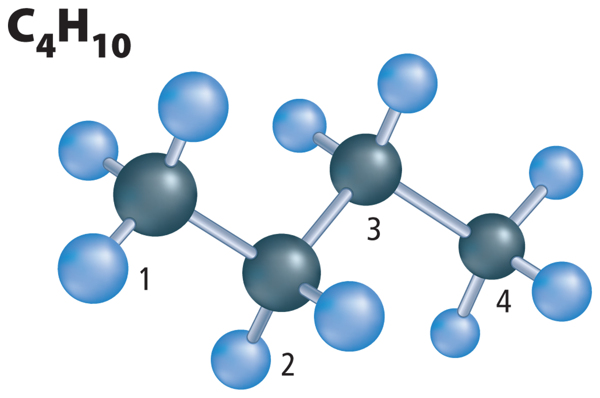
6. Examine each molecular formula. Which is propane?
A. CH3
B. C2H6
C. C3H8
D. C4H10
7. Explain why carbon is unique compared to other elements.
8. What is the maximum number of covalent bonds that carbon can form?
A. 1
B. 2
C. 3
D. 4
9. Which is the correct name for the following carbon compound pictured below?
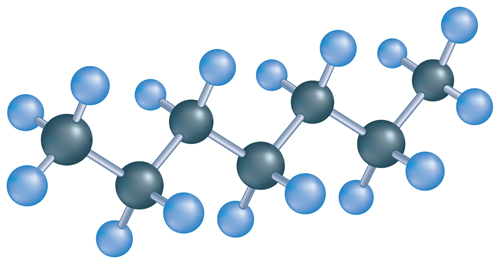

A. heptane
B. hexane
C. pentane
D. pentene
10. Which is true about the carbon compound cyclooctane?
A. It is a straight-chain hydrocarbon with eight carbon atoms and only single bonds.
B. It is a branched-chain hydrocarbon with eight carbon atoms and at least one double bond.
C. It is a ringed hydrocarbon with eight carbon atoms and at least one double bond.
D. It is a ringed hydrocarbon with eight carbon atoms and only single bonds.
11. Which is NOT a form of pure carbon?
A. diamond
B. fullerene
C. graphite
D. halide
Interpret Graphics
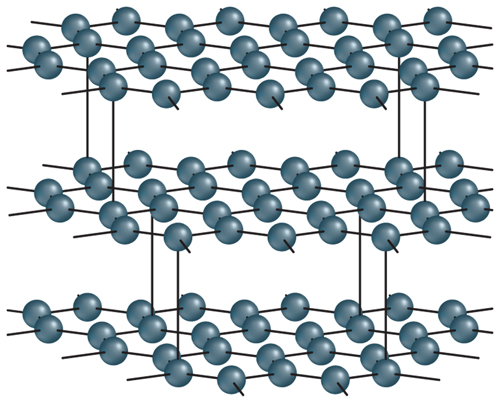
12. Identify Which form of pure carbon is represented in the diagram below?

13. Explain Copy and fill in the table below to explain how the four forms of carbon differ.

Critical Thinking
14. Explain why so many different compounds are made from carbon.
15. Draw the structural formula for pentane (C5H12).
16. Draw three isomers for pentane.
17. Differentiate between saturated and unsaturated hydrocarbons.
18. Compare and contrast the properties of diamond and graphite.
19. Create a poster that illustrates why hydrocarbons are present in so many different compounds.
20. Evaluate which of these formulas contains at least one double bond: C7H16, C4H8, C3H8. Explain your reasoning.






















0 comments:
Post a Comment