Make your own microscope with your phone
Does it run on batteries?
Flashes of light from fireflies dot summer evening skies in many parts of the United States. But, firefly light doesn’t come from batteries. Fireflies make light using a process called bioluminescence (bi oh lew muh NE cents). In this process, chemicals in the firefly’s body combine in a two-step process and make new chemicals and light.
2
Changes in Matter
When you put liquid water in a freezer, it changes to solid water, or ice. When you pour brownie batter into a pan and bake it, the liquid batter changes to a solid, too. In both cases, a liquid changes to a solid. Are these changes the same?
Physical Changes
Recall that matter can undergo two types of changes—chemical or physical. A physical change does not produce new substances. The substances that exist before and after the change are the same, although they might have different physical properties. This is what happens when liquid water freezes. Its physical properties change from a liquid to a solid, but the water, H2O, does not change into a different substance. Water molecules are always made up of two hydrogen atoms bonded to one oxygen atom regardless of whether they are solid, liquid, or gas.
Chemical Changes
Recall that during a chemical change, one or more substances change into new substances. The starting substances and the substances produced have different physical and chemical properties. For example, when brownie batter bakes, a chemical change occurs. Many of the substances in the baked brownies are different from the substances in the batter. As a result, baked brownies have physical and chemical properties that are different from those of brownie batter.
A chemical change also is called a chemical reaction. These terms mean the same thing. A chemical reaction is a process in which atoms of one or more substances rearrange to form one or more new substances. In this lesson, you will read what happens to atoms during a reaction and how these changes can be described using equations.
1.  Reading Check What types of properties change during a chemical reaction?
Reading Check What types of properties change during a chemical reaction?
 Reading Check What types of properties change during a chemical reaction?
Reading Check What types of properties change during a chemical reaction?
5
Signs of a Chemical Reaction
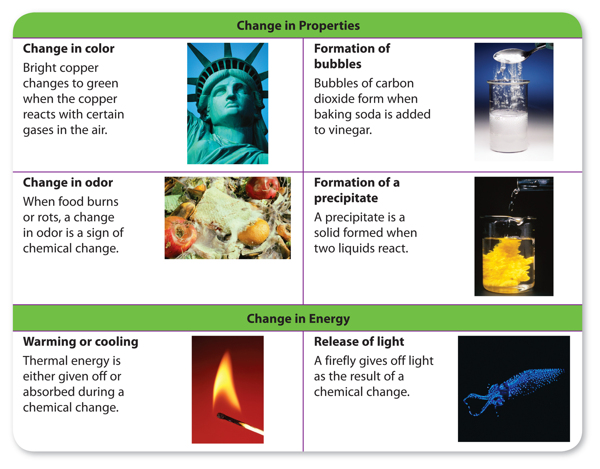
How can you tell if a chemical reaction has taken place? You have read that the substances before and after a reaction have different properties. You might think that you could look for changes in properties as a sign that a reaction occurred. In fact, changes in the physical properties of color, state of matter, and odor are all signs that a chemical reaction might have occurred. Another sign of a chemical reaction is a change in energy. If substances get warmer or cooler or if they give off light or sound, it is likely that a reaction has occurred. Some signs that a chemical reaction might have occurred are shown in Figure 1.
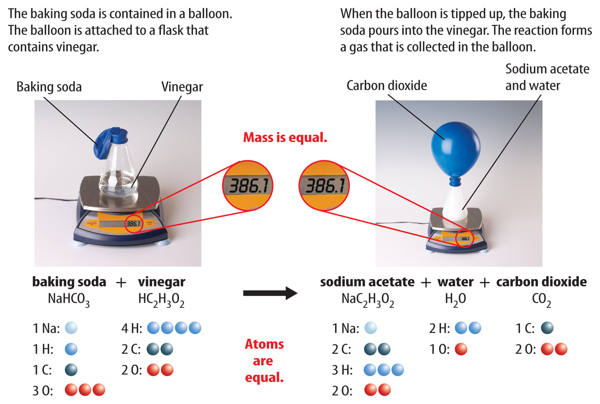
However, these signs are not proof of a chemical change. For example, bubbles appear when water boils. But, bubbles also appear when baking soda and vinegar react and form carbon dioxide gas. How can you be sure that a chemical reaction has taken place? The only way to know is to study the chemical properties of the substances before and after the change. If they have different chemical properties, then the substances have undergone a chemical reaction.
1. Key Concept Check What are some signs that a chemical reaction might have occurred?
Figure 1 You can detect a chemical reaction by looking for changes in properties and changes in energy of the substances that reacted.
What happens in a chemical reaction?
During a chemical reaction, one or more substances react and form one or more new substances. How are these new substances formed?
Atoms Rearrange and Form New Substances
To understand what happens in a reaction, first review substances. Recall that there are two types of substances—elements and compounds. Substances have a fixed arrangement of atoms. For example, in a single drop of water, there are trillions of oxygen and hydrogen atoms. However, all of these atoms are arranged in the same way—two atoms of hydrogen are bonded to one atom of oxygen. If this arrangement changes, the substance is no longer water. Instead, a different substance forms with different physical and chemical properties. This is what happens during a chemical reaction. Atoms of elements or compounds rearrange and form different elements or compounds.
Bonds Break and Bonds Form
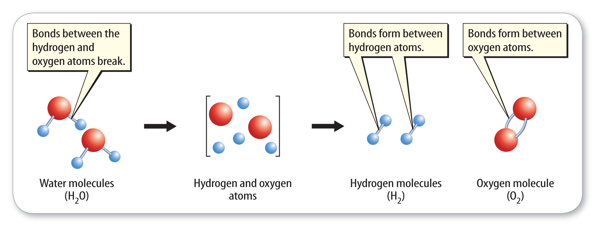
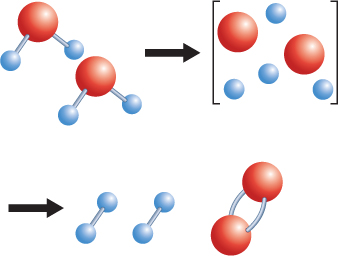
How does the rearrangement of atoms happen? Atoms rearrange when chemical bonds between atoms break. Recall that constantly moving particles make up all substances, including solids. As particles move, they collide with one another. If the particles collide with enough energy, the bonds between atoms can break. The atoms separate, rearrange, and new bonds can form. The reaction that forms hydrogen and oxygen molecules from water is shown in Figure 2.
Adding electric energy to water molecules can cause this reaction. The added energy causes bonds between the hydrogen atoms and the oxygen atoms to break. After the bonds between the atoms in water molecules break, new bonds can form between pairs of hydrogen atoms and between pairs of oxygen atoms.
1. Key Concept Check What happens to atoms during a chemical reaction?
Figure 2 Notice that no new atoms are created in a chemical reaction. The existing atoms rearrange and form new substances.
1
REVIEW VOCABULARY
chemical bond
an attraction between atoms when electrons are shared, transferred, or pooled
an attraction between atoms when electrons are shared, transferred, or pooled
11
Chemical Equations
Suppose your teacher asks you to produce a specific reaction in your science laboratory. How might your teacher describe the reaction to you? He or she might say something such as “react baking soda and vinegar to form sodium acetate, water, and carbon dioxide.” It is more likely that your teacher will describe the reaction in the form of a chemical equation. A chemical equation is a description of a reaction using element symbols and chemical formulas. Element symbols represent elements. Chemical formulas represent compounds.
Element Symbols
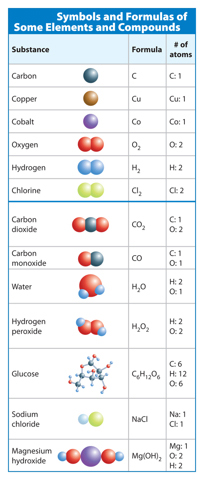
Recall that symbols of elements are shown in the periodic table. For example, the symbol for carbon is C. The symbol for copper is Cu. Each element can exist as just one atom. However, some elements exist in nature as diatomic molecules—two atoms of the same element bonded together. A formula for one of these diatomic elements includes the element’s symbol and the subscript 2. A subscript describes the number of atoms of an element in a compound. Oxygen (O2) and hydrogen (H2) are examples of diatomic molecules. Some element symbols are shown below the blue line in Table 1.
Table 1 Symbols and subscripts describe the type and number of atoms in an element or a compound.
 Visual Check Describe the number of atoms in each element in the following: C, Co, CO, and CO2.
Visual Check Describe the number of atoms in each element in the following: C, Co, CO, and CO2.
Chemical Formulas
When atoms of two or more different elements bond, they form a compound. Recall that a chemical formula uses elements’ symbols and subscripts to describe the number of atoms in a compound. If an element’s symbol does not have a subscript, the compound contains only one atom of that element. For example, carbon dioxide (CO2) is made up of one carbon atom and two oxygen atoms. Remember that two different formulas, no matter how similar, represent different substances. Some chemical formulas are shown below the blue line in Table 1.
Writing Chemical Equations
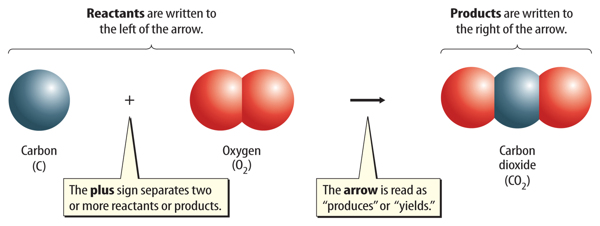
A chemical equation includes both the substances that react and the substances that are formed in a chemical reaction. The starting substances in a chemical reaction are reactants. The substances produced by the chemical reaction are products. Figure 3 shows how a chemical equation is written.
Chemical formulas are used to describe the reactants and the products. The reactants are written to the left of an arrow, and the products are written to the right of the arrow. Two or more reactants or products are separated by a plus sign. The general structure for an equation is:
reactant + reactant → product + product
When writing chemical equations, it is important to use correct chemical formulas for the reactants and the products. For example, suppose a certain chemical reaction produces carbon dioxide and water. The product carbon dioxide would be written as CO2 and not as CO. CO is the formula for carbon monoxide, which is not the same compound as CO2. Water would be written as H2O and not as H2O2, the formula for hydrogen peroxide.
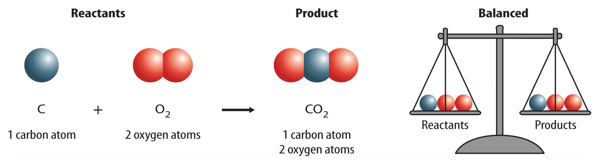
Figure 3 An equation is read much like a sentence. This equation is read as “carbon plus oxygen produces carbon dioxide.”
1
 MiniLab: How does an equation represent a reaction?
MiniLab: How does an equation represent a reaction?

Sulfur dioxide (SO2) and oxygen (O2) react and form sulfur trioxide (SO3). How does an equation represent the reaction?
1. Read and complete a lab safety form.
2. Use yellow modeling clay to model two atoms of sulfur. Use red modeling clay to model six atoms of oxygen.
3. Make two molecules of SO2 with a sulfur atom in the middle of each molecule. Make one molecule of O2. Sketch the models in your Science Journal.
4. Rearrange atoms to form two molecules of SO3. Place a sulfur atom in the middle of each molecule. Sketch the models in your Science Journal.
Analyze and Conclude
1. Identify the reactants and the products in this chemical reaction.
2. Write a chemical equation for this reaction.
3. Explain What do the letters represent in the equation? The numbers?
4. Key Concept In terms of chemical bonds, what did you model by pulling molecules apart and building new ones?
14
Conservation of Mass
A French chemist named Antoine Lavoisier (AN twan · luh VWAH see ay) (1743–1794) discovered something interesting about chemical reactions. In a series of experiments, Lavoisier measured the masses of substances before and after a chemical reaction inside a closed container. He found that the total mass of the reactants always equaled the total mass of the products. Lavoisier’s results led to the law of conservation of mass. The law of conservation of mass states that the total mass of the reactants before a chemical reaction is the same as the total mass of the products after the chemical reaction.
Atoms are conserved.
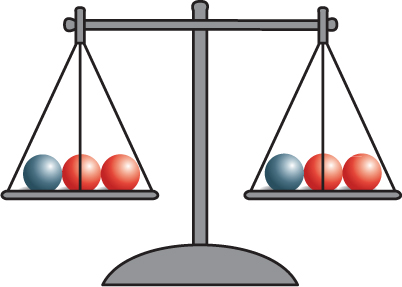
The discovery of atoms provided an explanation for Lavoisier’s observations. Mass is conserved in a reaction because atoms are conserved. Recall that during a chemical reaction, bonds break and new bonds form. However, atoms are not destroyed, and no new atoms form. All atoms at the start of a chemical reaction are present at the end of the reaction. Figure 4 shows that mass is conserved in the reaction between baking soda and vinegar.
1. Key Concept Check What happens to the total mass of the reactants in a chemical reaction?
Figure 4 As this reaction takes place, the mass on the balance remains the same, showing that mass is conserved.
Is an equation balanced?
How does a chemical equation show that atoms are conserved? An equation is written so that the number of atoms of each element is the same, or balanced, on each side of the arrow. The equation showing the reaction between carbon and oxygen that produces carbon dioxide is shown below. Remember that oxygen is written as O2 because it is a diatomic molecule. The formula for carbon dioxide is CO2.
Is there the same number of carbon atoms on each side of the arrow? Yes, there is one carbon atom on the left and one on the right. Carbon is balanced. Is oxygen balanced? There are two oxygen atoms on each side of the arrow. Oxygen also is balanced. The atoms of all elements are balanced. Therefore, the equation is balanced.
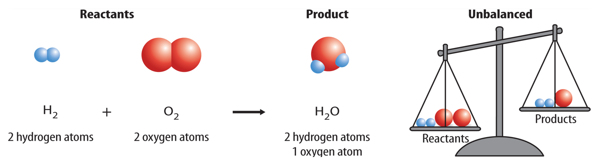
You might think a balanced equation happens automatically when you write the symbols and formulas for reactants and products. However, this usually is not the case. For example, the reaction between hydrogen (H2) and oxygen (O2) that forms water (H2O) is shown below.
Count the number of hydrogen atoms on each side of the arrow. There are two hydrogen atoms in the product and two in the reactants. They are balanced. Now count the number of oxygen atoms on each side of the arrow. Did you notice that there are two oxygen atoms in the reactants and only one in the product? Because they are not equal, this equation is not balanced. To accurately represent this reaction, the equation needs to be balanced.
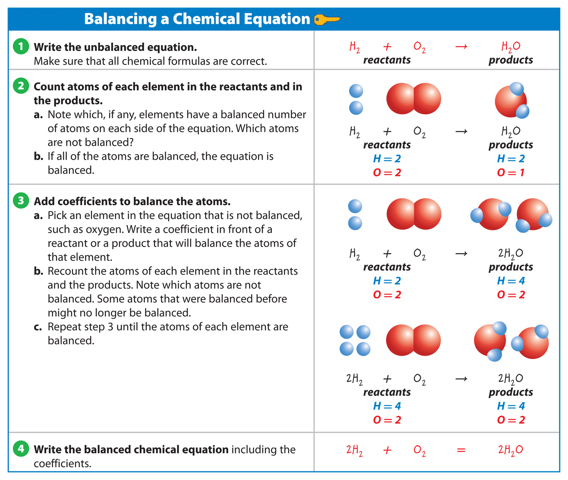
Balancing Chemical Equations
When you balance a chemical equation, you count the atoms in the reactants and the products and then add coefficients to balance the number of atoms. A coefficient is a number placed in front of an element symbol or chemical formula in an equation. It is the number of units of that substance in the reaction. For example, in the formula 2H2O, the 2 in front of H2O is a coefficient. This means that there are two molecules of water in the reaction. Only coefficients can be changed when balancing an equation. Changing subscripts changes the identities of the substances that are in the reaction.
If one molecule of water contains two hydrogen atoms and one oxygen atom, how many H and O atoms are in two molecules of water (2H2O)? Multiply each by 2.

When no coefficient is present, only one unit of that substance takes part in the reaction. Table 2 shows the steps of balancing a chemical equation.
Table 2 Balancing a Chemical Equation
 Visual Check In row 2 above, which element is not balanced? In the top of row 3, which element is not balanced?
Visual Check In row 2 above, which element is not balanced? In the top of row 3, which element is not balanced?
1
WORD ORIGIN
product
from Latin producere, means “bring forth”
from Latin producere, means “bring forth”
Lesson Review
Visual Summary
A chemical reaction is a process in which bonds break and atoms rearrange, forming new bonds.
A chemical equation uses symbols to show reactants and products of a chemical reaction.
The mass and the number of each type of atom do not change during a chemical reaction. This is the law of conservation of mass.
What do you think NOW?
You first read the statements below at the beginning of the lesson.
1. If a substance bubbles, you know a chemical reaction is occurring.
2. During a chemical reaction, some atoms are destroyed and new atoms are made.
Did you change your mind about whether you agree or disagree with the statements? Rewrite any false statements to make them true.
Lesson Assessment
Use Vocabulary
1. Define reactants and products.
Understand Key Concepts
2. Which is a sign of a chemical reaction?
A. chemical properties change
B. physical properties change
C. a gas forms
D. a solid forms
3. Explain why subscripts cannot change when balancing a chemical equation.
4. Infer Is the reaction below possible? Explain why or why not.
H2O + NaOH → NaCl + H2
H2O + NaOH → NaCl + H2
5. How many carbon atoms react in this equation?
2C4H10 + 13O2 → 8CO2 + 10H2O
2C4H10 + 13O2 → 8CO2 + 10H2O
A. 2
B. 4
C. 6
D. 8
6. The chemical equation below is unbalanced.
Zn + HCl → ZnCl2 + H2
Which is the correct balanced chemical equation?
Zn + HCl → ZnCl2 + H2
Which is the correct balanced chemical equation?
A. Zn + H2Cl2 → ZnCl2 + H2
B. Zn + HCl → ZnCl + H
C. 2Zn + 2HCl → ZnCl2 + H2
D. Zn + 2HCl → ZnCl2 + H2
7. When iron combines with oxygen gas and forms rust, the total mass of the products
A. depends on the reaction conditions.
B. is less than the mass of the reactants.
C. is the same as the mass of the reactants.
D. is greater than the mass of the reactants.
Interpret Graphics
8. Describe the reaction below by listing the bonds that break and the bonds that form.
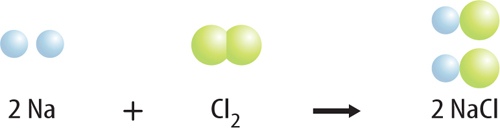
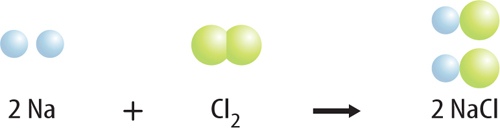
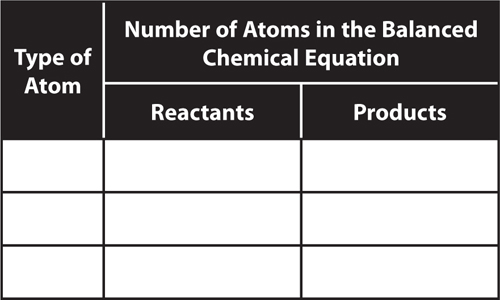
9. Interpret Copy and complete the table to determine if this equation is balanced:
CH4 + 2O2 → CO2 + 2H2O
Is this reaction balanced? Explain.
CH4 + 2O2 → CO2 + 2H2O
Is this reaction balanced? Explain.

Critical Thinking
10. Balance this chemical equation. Hint: Balance Al last.
11. Analyze A student observed a chemical reaction and collected the following data:
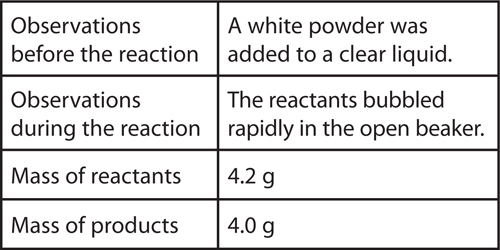
The student concludes that mass was not conserved in the reaction. Explain why this is not a valid conclusion. What might explain the difference in mass?

The student concludes that mass was not conserved in the reaction. Explain why this is not a valid conclusion. What might explain the difference in mass?
12. Explain Observations How did the discovery of atoms explain the observation that the mass of the products always equals the mass of the reactants in a reaction?
13. Explain how atoms and energy are conserved in a chemical reaction.
Writing in Science
14. Write Instructions that explain the steps in balancing a chemical equation. Use the following equation as an example.
MnO2 + HCl → MnCl2 + H2O + Cl2
MnO2 + HCl → MnCl2 + H2O + Cl2



2 comments:
Thank you from one science teacher to another! You really helped me figure out the flow of this kind of lesson.
I have a way better understand now! Thank you very much, Mr./Mrs./Ms. Sandoval.
Post a Comment